what quantum number specify these subshells|3.2: Quantum Numbers for Atomic Orbitals : Tagatay We can summarize the relationships between the quantum numbers and the number of subshells and orbitals as follows (Table 6.5.1): Each principal shell has n . On the campaign map, put them into a port and then there will be a repair icon to the left of the boat icons. Press that to repair them. "Tether even a roasted chicken."
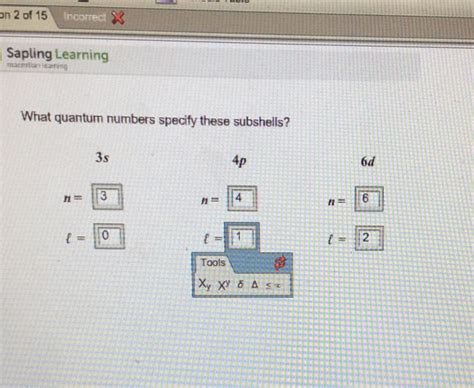
PH0 · What quantum numbers specify these subshells?
PH1 · What quantum numbers specify these subshells 5s 6p 4d?
PH2 · What quantum numbers specify these subshells
PH3 · What quantum numbers specify subshells?
PH4 · What Quantum Numbers Specify These Subshells?
PH5 · What Quantum Numbers Specify These Subshells 6s?
PH6 · Quantum numbers (video)
PH7 · Quantum Numbers for Atoms
PH8 · Quantum Numbers
PH9 · Flexi answers
PH10 · 3.2: Quantum Numbers for Atomic Orbitals
Apostas na NBA com o Odds Shark Apostar na NBA é uma das melhores opções de lucros para quem é fã da liga de basquete profissional dos Estados Unidos e curte investir nos chamados mercados esportivos. Com muitos jogos para fazer seus palpites, uma enorme variedade de apostas e cotações muito vantajosas também nos palpites de .
what quantum number specify these subshells*******The number is the "n" quantum number and the orbital type s,p,d,f are the "l" quantum numbers 0,1,2,3 respectively. But, what quantum numbers specify these subshells? The answer lies within the realm of quantum mechanics. Quantum numbers are essential tools for .
In atoms, there are a total of four quantum numbers: the principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum .
3.2: Quantum Numbers for Atomic Orbitals Quantum Numbers: Principal Quantum Number. Quantum Numbers: Magnetic Quantum Number. Quantum Numbers: Spin Quantum Number. Quantum Numbers: Number . We can summarize the relationships between the quantum numbers and the number of subshells and orbitals as follows (Table 6.5.1): Each principal shell has n .
The quantum number that specifies the subshells in an atom is the azimuthal quantum number, often denoted as "l" (lowercase L). The azimuthal quantum number .The shape of a subshell is described by the quantum number ℓ. ℓ can be any positive integer from 0 to n-1. So, there is the possibility of many subshells of many shapes. .
Shells and Subshells of Orbitals. Orbitals that have the same value of the principal quantum number form a shell.Orbitals within a shell are divided into subshells that have the same value of the angular quantum . The first three quantum numbers are called the principal quantum number (n), the azimuthal quantum number (ℓ), and the magnetic quantum number (m). The .
Step 1. Quantum numbers are a set of four parameters that describe the unique characteristics and properties. What quantum numbers specify these subshells?what quantum number specify these subshellsThe quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. They are: 1. PRINCIPAL QUANTUM NUMBER (n) - Represents the main energy level, or shell, occupied by an electron. It is always a positive integer, that is n = 1, 2, 3 .An orbital is a region around an atom's nucleus where electrons are likely to be found. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are used to describe the orbitals, and compare Bohr model orbits with the quantum mechanical model of atom.
In this article, we will discuss how to calculate quantum numbers for subshells with 6p orbitals. Step-by-Step Guide to Calculating Principal Quantum Number for 6p Subshells. The principal quantum number (represented by n) specifies the energy level at which the electron resides. To calculate the value of n for the 6p subshell, follow .
What quantum numbers specify these sub shells? For 3s n=? l=? / 5p n=? l=? / 3d n=? l=? Here’s the best way to solve it. Who are the experts? Experts have been vetted by Chegg as specialists in this subject. Expert-verified.Did you know that quantum numbers are used to specify subshells in atoms? For the subshell 2s, the quantum numbers are n=2 and l=0. For the subshell 3p, it is n=3 and l=1. Lastly, for the subshell 6d, the quantum numbers are n=6 and l=2. These numbers provide important information about the energy levels and orbital shapes within an atom. The 6s subshell is a complex system of electrons that determines the chemical and physical properties of an atom. Understanding the quantum numbers that specify these subshells is key to understanding their behavior. One such number, called the spin quantum number, affects subshells by determining the direction in which the .What quantum numbers specify subshells? Flexi Says: The quantum number that specifies the subshells in an atom is the azimuthal quantum number, often denoted as "l" (lowercase L). The azimuthal quantum number determines the shape of the electron's orbital and indirectly identifies the subshell. The 6s subshells are identified by the four quantum numbers n, l, ml, and ms. The principal quantum number (n) determines the size of the orbital and the energy of the electron. It can take on any integer values from 1 to infinity. For the 6s subshell, n = 6. The azimuthal quantum number (l) determines the shape of the orbital.
Quantum numbers are used to describe the state of an electron in an atom. They specify the subshells as follows: 1. For the 7s subshell: n (principal quantum number) = 7, which indicates the energy level and distance from the nucleus. ℓ (azimuthal quantum number) = 0, which defines the subshell shape (s, p, d, or f).

There are 2 steps to solve this one. To start solving, it's necessary to understand that quantum numbers are used to specify the position and energy of an electron in an atom with the Principal Quantum Number (n) indicating the shell the electron belongs to, determining the size and the energy of the orbital while the Azimuthal Quantum number .What quantum numbers specify these subshells? 2𝑠. 𝑛= ℓ= 6𝑝. 𝑛= ℓ= 6𝑑. 𝑛= ℓ= Final answer: Subshells in quantum mechanics are specified by three quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), and the magnetic quantum number (m). These quantum numbers define the orbital size, shape, and orientation. For example, 2p⁴ refers to four electrons in a p . To specify the subshells in an atom, we use a notation that includes the principal quantum number (n) and the azimuthal quantum number (l).The principal quantum number (n) determines the energy level of an electron, and it can take on any positive integer value.The azimuthal quantum number (l) determines the shape of the .what quantum number specify these subshells 3.2: Quantum Numbers for Atomic Orbitals The position of an electron in an atom is specified using four quantum numbers: 1) Principal quantum . View the full answer Step 2. Unlock. Answer. Unlock. Previous question Next question. Transcribed image text: What quantum numbers specify these subshells? 3s 5 p n = n = n = l = l = l =.The Four Electronic Quantum Numbers. Quantum numbers designate specific shells, subshells, orbitals, and spins of electrons. This means that they describe completely the characteristics of an electron in an atom, i.e., they describe each unique solution to the Schrödinger equation, or the wave function, of electrons in an atom.There are a total of . So, the principal quantum number, n, for the 5p-subshell is n = 5. Now, the any p-subshell is characterized by l = 1. Similarly, any s-subshell is characterized by l = 0, any d-subshell by l = 2, and so on. Therefore, the value of angula momentum quantum number will be l = 1. n=5 l=1 As you know, quantum numbers are sused to describe the .Question: What quantum numbers specify these subshells 2s N= L= 4b N= L= 5d N= L= What quantum numbers specify these subshells. 2s. N= L= 4b. N= L= 5d. N= L= There are 2 steps to solve this one. Who are the experts? Experts have been vetted by Chegg as specialists in this subject. Expert-verified.
True aficionados look for the most exciting eye colors – the odd-eyed! Also known as heterochromia, it means eyes of different colors. Khao Manees generally have oval-shaped green, gold, or blue eyes. But the most valuable cats (in terms of cost) have a combination – sometimes blue and green, occasionally blue and gold. . Something to .
what quantum number specify these subshells|3.2: Quantum Numbers for Atomic Orbitals